What Is Hardness Of Water. Hardness in water can indicate high concentrations of undissolved minerals. Perhaps you have on occassion noticed mineral deposits on your cooking dishes, or rings of insoluble.

Water hardness is a value that reflects the amount of dissolved calcium, magnesium, and iron salts in water.
What we are doing to protect customers while delivering services as normal plus information about registering for Priority Services.
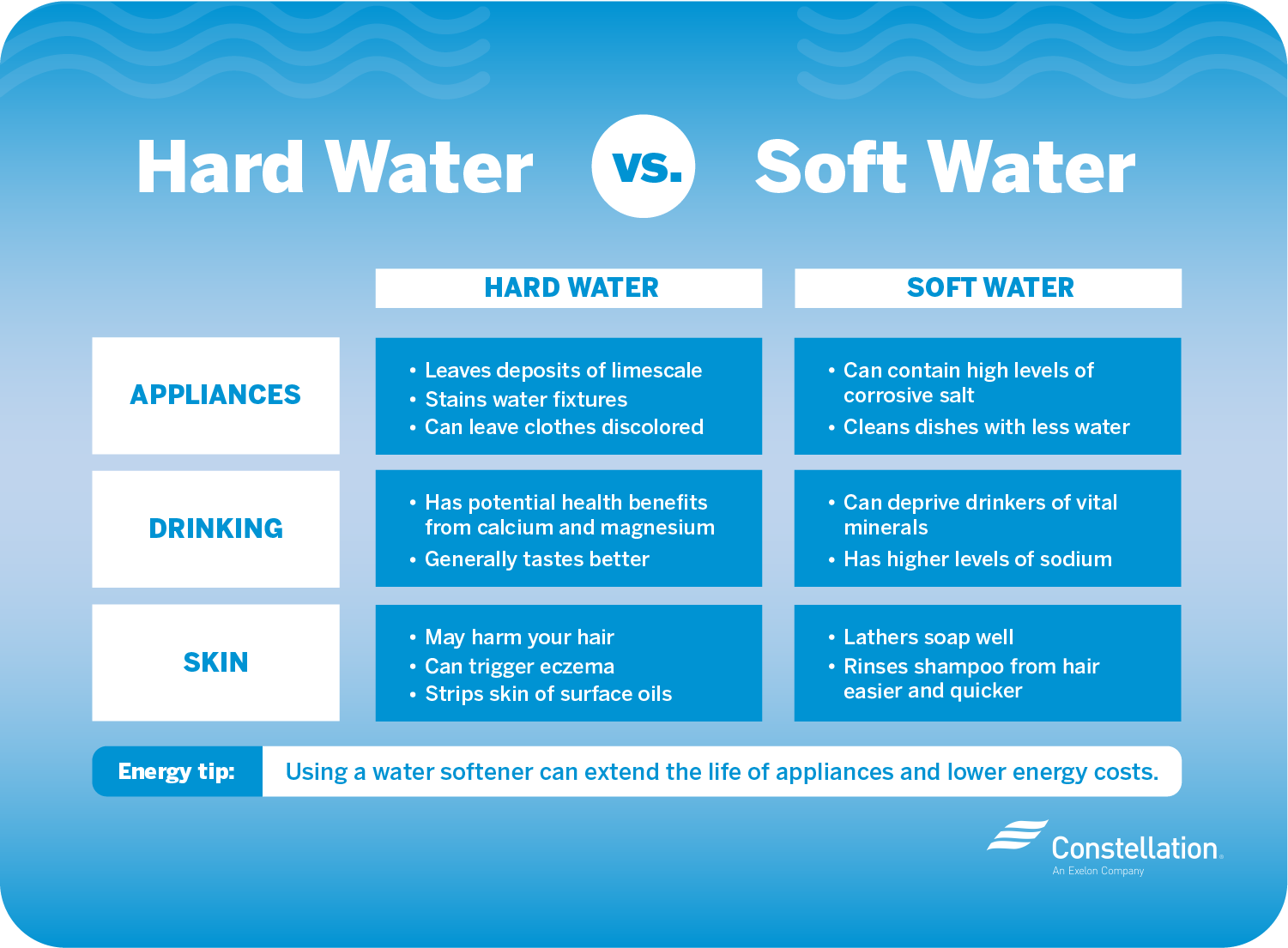
You've probably heard the terms "hard water" and "soft water." You may wonder what determines the hardness or softness of water and whether one type of water is healthier or safer to drink than the other. Water "hardness" refers to the amount calcium and magnesium dissolved in the water. Hard water often produces a noticeable deposit of precipitate (e.g. insoluble metals, soaps or salts) in containers, including "bathtub ring". Usually, hard water contains a high amount of calcium and magnesium. Water hardness varies throughout the United States. Yes, in adequate concentrations, calcium and magnesium have a positive effect on human health and also on plants.
When washing laundry, observe the dosage. It is a measure of the quantity of divalent ions (for this discussion, salts with two positive charges) such as calcium, magnesium and/or iron in water. Higher levels are often found in municipal water, which is often "softened"—particularly in the United States—to be used at home. Water hardness is due to the presence of calcium ions, and to a lesser extent, magnesium, iron, and manganese. The concentration of certain minerals is what creates the "hardness" of water. Water hardness is the traditional measure of the capacity of water to react with soap, hard water requiring considerably more soap to produce a lather.
Carbonate and bicarbonate ions are responsible for this type of water hardness. Water is an excellent solvent and readily dissolves minerals it comes The hardness of water is referred to by three types of measurements: grains per gallon, milligrams per liter (mg/L), or parts per million (ppm). It is also known as temporary hardness because it removes from water when we boil the water.