What Is Soft Water And Hard Water. The American National Standards defines soft water as containing less. Although water is typically crystal clear, it contains minerals and chemicals.

While soft water examples include rainfall when it is clean and has not Answer.
So, what is the difference between soft water vs. hard water and how does it affect you?
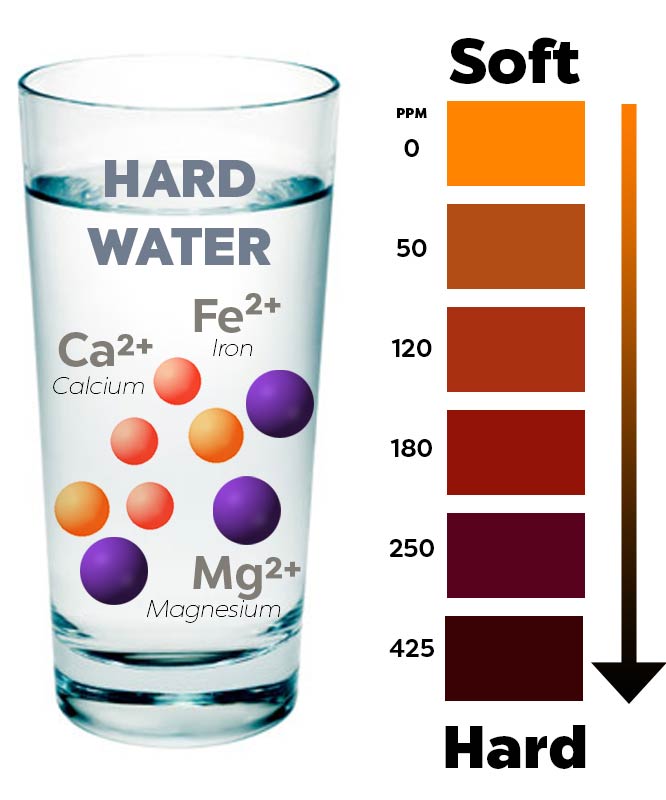
Hard water is water that has high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone. For many, the hard water versus soft water debate boils down to costs. The term 'hard' implies the water contains a high level of minerals. Hard water refers to water that has a substantial concentration of calcium and magnesium. Hardness also depends on the pH and temperature of the water.
Soft water is treated water in which the only cation (positively charged Hard water can be softened (have its minerals removed) by treating it with lime or by passing it over an ion exchange resin. Hard water has a high mineral content, meaning it's packed with minerals like calcium and magnesium. When water boils down, the major difference between hard and soft water can best be seen while doing daily housework. Water is a vital element that is needed by all living things. Hard water is water with a high mineral content, particularly ions of calcium and magnesium. Places rich in rock such as slate or granite, which doesn't dissolve in water very easily, will have softer water.
Soft water feels softer and flows differently from hard water. Hard water is any water containing an appreciable quantity of dissolved minerals. As the name suggests, this system turns water from being hard to being soft.