Salt Free Water Softener System. Soft water alleviates the stress of endlessly. If you're in the market for a salt-free water softener, unfortunately, you are out of luck.

Before we can understand how a salt-free.
Salt softeners seem to produce a more slippery feel for water.
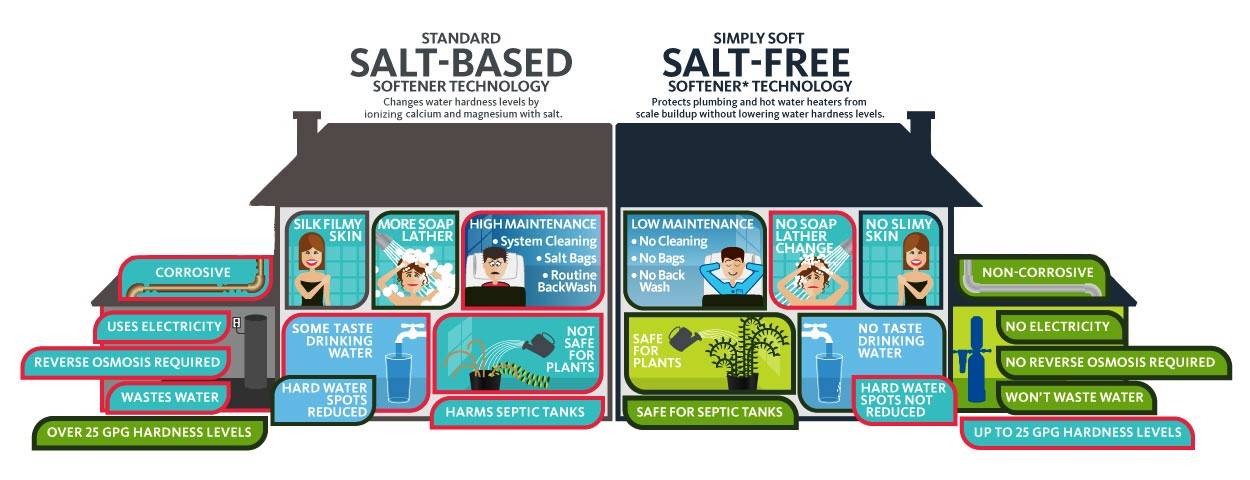
Notice that we did not ever call them "salt-free water softeners." You might wonder why that is… and I will get to that later. A salt-free water softener does not exist. Polyphosphate-filtering salt-free water softeners use safe chemicals (polyphosphates) to coat mineral grains so that they can't stick to pipes or other surfaces in your house. Salt free water softening systems don't actually make the water softer. Water softener salt removes minerals, including calcium and magnesium — which cause hard water — through a process called ionic exchange and replaces. Salt-free systems also reported to produce this but is a lot less.
Some water purists do not think that salt-free systems are real water softeners. Salt-free water softeners use the Template Assisted Crystallization process to turn hard water into soft water. The water softener system is capable of providing you very soft water and gives you an experience of a spa at home. Naturally, the form that the salt comes in is important for your water softening system. No salt, chemicals, or electricity, Easy to install. Soft water alleviates the stress of endlessly.
Salt-free softeners are also known as water conditioners because they do not actually "soften" the water; They condition (or neutralize) it. Salt-free water softeners use the Template Assisted Crystallization process to turn hard water into soft water. Salt-using water softening eliminates minerals such as calcium and magnesium that cause hardness through ionic exchange.