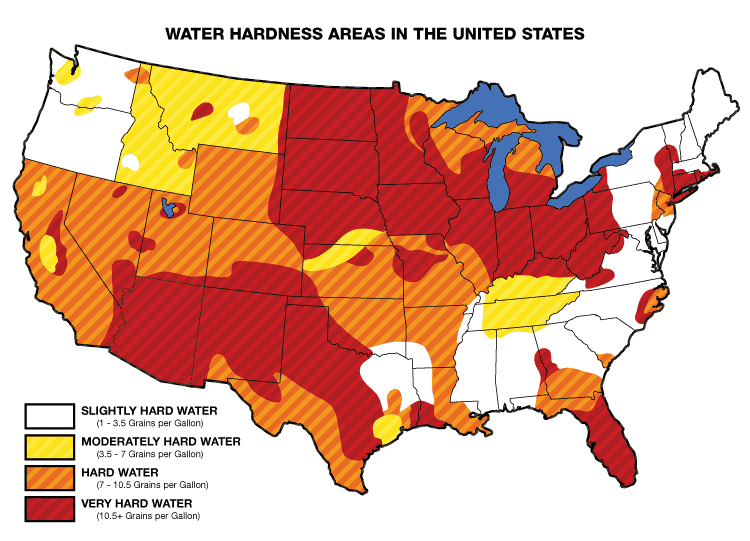
Hard Water And Soft Water Examples. Drinking hard water can have certain benefits to human health. The concentration of certain minerals is what creates the hardness of water.

Hard water and soft water contain many properties, including minerals and chemicals.
Give examples of hard water and soft water.
Soft water doesn't appear in nature as often as hard water does. It is mostly used for household purposes. Iron oxides or iron carbonates can give a reddish brown colouration to hard water deposits. It can help prevent residue buildup in plumbing. Hard water is formed when water percolates through deposits of limestone, chalk or gypsum which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates. Soft Water: The Differences Composition and Source of Hard Water and Soft Water.
Soft water is water in which dissolved metals have been exchanged for sodium ions. Water can be naturally soft, like rain water or it can be chemically softened to remove calcium and magnesium. Hard water is any water containing an appreciable quantity of dissolved minerals. Hard water has a higher concentration of these dissolved minerals. This logic is borne of the fact that the water simply isn't "Hard", usually on account of the rock it has passed across between the spring where it emerged from the ground in the water cycle and the faucet. The boiler, therefore, remains efficient and uses less energy to heat the water.
B—Temporal hard water precipitates calcium deposits, which build up along the coils. The difference between hard and soft water is in their composition. Hard water is formed when water percolates through deposits of limestone, chalk or gypsum which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates.