How To Precipitate Iron From Water. Mollusks have teeth of magnetite of goethite. In the case of acid water, the treatment could be supplemented by a correction of the pH.
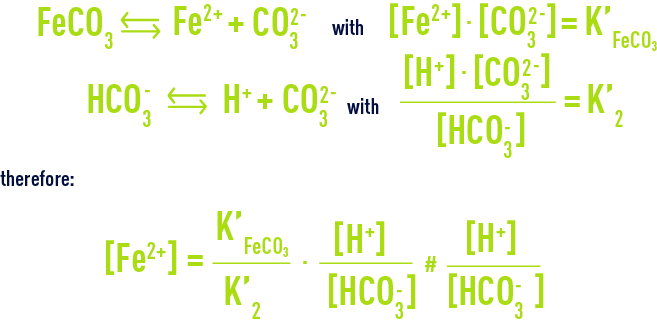
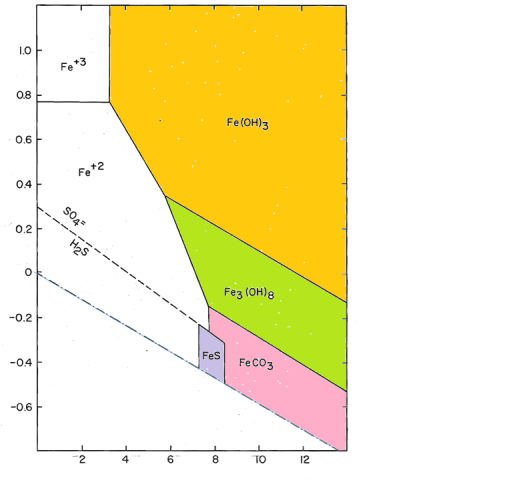
Do ferric and ferrous precipitate in an equal pH?
An effective technique of removing iron cheaply is by aeration.
This is the process that the best iron filters use. Water softeners are designed to remove hard water minerals, like calcium and magnesium, from your water. Chlorination as an oxidizing agent to remove organiciron rich water. For the best results, you may need both a water softener and an iron filter. An iron filter is specifically designed to tackle iron. Aerate the water to oxidize the iron or manganese, then allow the precipitate to settle out before you use the water for irrigation.
Sediment filters allow water to flow freely through them while preventing solid particulate matter from entering the household plumbing. For higher levels of iron in the water, some softeners require a special blend of salt that contains additives to help remove the iron from the resin. An iron filter is specifically designed to tackle iron. Organic iron is a compound formed from an organic acid and iron. Water softeners work by removing ferrous iron ions and replacing them with sodium. Chlorine or copper compounds may be needed to control algae growth in the reservoir or basin.
The presence of iron in ground water is a direct result of its natural existence in underground rock formations and precipitation water that infiltrates through these formations. Aerate the water to oxidize the iron or manganese, then allow the precipitate to settle out before you use the water for irrigation. For higher levels of iron in the water, some softeners require a special blend of salt that contains additives to help remove the iron from the resin.